


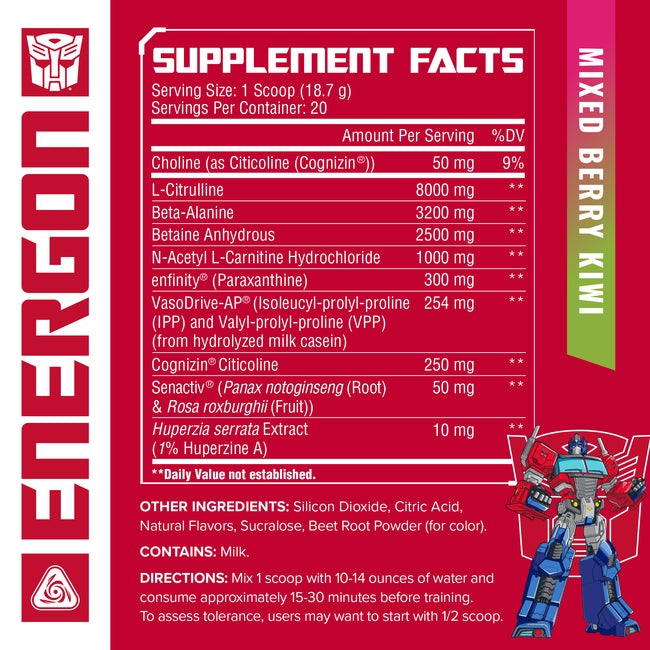




L-Citrulline
Citrulline is a non-essential, non-protein amino acid that forms during the urea cycle and forms ornithine when combined with carbon dioxide. Citrulline is also a critical source of endogenous (natural) arginine, as it is rapidly and efficiently converted to arginine in the vascular endothelium and other tissues.
Citrulline’s benefits have been shown to be greater than its parent compound. While arginine undergoes direct hepatic (liver) metabolism through the enzyme arginase, citrulline bypasses hepatic metabolism entirely and it is delivered straight to the bloodstream. The result is that gut absorption and plasma (blood) bioavailability studies comparing citrulline and arginine have shown two things. First, citrulline is less readily destroyed and has greater absorption than arginine. Second, citrulline supplementation increases arginine levels more effectively than arginine supplementation itself.
This translates to promising results. For example, animal studies show a significant increase in anaerobic performance at a 250mg/kg/day serving of citrulline, while studies in humans implicate citrulline in both aerobic and anaerobic performance increases. As a critical part of the urea cycle, citrulline’s performance benefits are thought to be a result of its role in ammonia clearance. Citrulline is implicated in reducing the oxygen cost of muscle processes, along with increasing the rate of post-exercise ATP and phosphocreatine replenishment. As ATP and phosphocreatine are the body’s ‘exercise fuel,’ this may result in citrulline delaying time to exhaustion in aerobic and anaerobic exercise.
Beta Alanine
Carnosine is a bit of an odd duck: we know that it is crucial for muscle function, and that dietary sources of carnosine are essential, but we don’t know precisely how it works. Moreover, for decades, we had no idea how to increase intramuscular concentrations, as exogenous carnosine sources degraded in the body so fast as to be effectively useless.
Enter beta-alanine. Simply a different iteration of one of the amino acids that comprises carnosine itself (alanine), beta-alanine has proven to be the most effective means of significantly increasing intramuscular concentrations of carnosine – and therefore of promoting all of carnosine’s various beneficial effects on muscle performance. If that weren’t enough, beta-alanine has also demonstrated beneficial physiological effects independent of its parent compound. To understand why, though, we need to first understand some of the basics behind carnosine itself.
Carnosine, a cytoplasmic dipeptide synthesized from the precursors L-histidine and l-alanine, is present in high concentrations in skeletal muscle and plays a pivotal role as a “chemical buffer” in myocytes (muscle cells). It has long been known that carnosine concentrations are highest in glycolytic, rather than oxidative muscle fibers (roughly speaking, explosive vs., endurance muscle fibers, respectively), and thus long hypothesized that this amino acid is required for sustained performance during supramaximal exercise. Recent research demonstrates that carnosine exerts its physiological effects in long hypoxic (low oxygen) drives by functioning as a high-capacity pH buffer in skeletal muscle, preventing the pH ratio of plasma from dropping too low – and therefore preventing crucial pH-dependent processes such as protein synthesis from being inhibited by acidosis.
Despite its critical role in skeletal muscle anaerobic performance, intramyocellular synthesis of carnosine is rate-limited by the availability of l-alanine. Unfortunately, the majority of literature demonstrates that attempting to increase intramuscular levels of carnosine via either direct carnosine or alanine supplementation is largely ineffective due to carnosine/alanine pharmacokinetics. Enter beta-alanine. Research with beta-alanine demonstrates consistent and dose-dependent increases to intramuscular carnosine concentrations with beta-alanine supplementation, with certain studies showing an increase of 40-60% with chronic administration. These same literatures reveal a synergistic effect of exercise on beta-alanine supplementation, whereby the muscle adaptive changes associated with resistance training promote further intramuscular carnosine production in response to beta-alanine supplementation.
In simpler language, this essentially means that beta-alanine is a dietary supplement that promotes its own effects in combination with exercise. As you exercise, you simultaneously intensify beta-alanine’s physiological actions – both directly, as well as in the production of intramuscular carnosine. Once ingested, beta-alanine’s exercise-specific beneficial activity is well-established. Elevation of intramuscular carnosine content via beta-alanine supplementation has been shown to improve performance in the following ways.
- Both acute and chronic increases in total work capacity, measured by total volume during exercise sessions.
- Highly significant increases to TTE (total time to exhaustion), one of the most accurate and comprehensive measures of endurance. In various trials, beta-alanine supplementation has been shown to increase TTE by upwards of 20%.
Increases to total muscle power output in both acute and chronic trials, suggesting that beta-alanine’s most significant benefit is to those engaging in power-dependent resistance training.
In total, a significant body of research exists to suggest that beta-alanine may significantly increase muscle power output, strength, training volume and output, overall performance in hypoxic (oxygen-deprived) conditions and peak VO2 max (oxygen holding capacity).
These myriad benefits make beta-alanine both one of the most-studied, and most well-rounded dietary supplements. Beta-alanine not only has direct, actionable physiological effects, but also promotes critical muscle physiologic adaptations that promote its own effects.
Betaine Anhydrous
Betaine (trimethyl glycine) is found naturally in most living organisms. It is well known to protect non-mammalian animal life in conditions of osmotic stress (a rapid change in the amount of solute surrounding a cell), in addition to functioning as an osmolyte in mammalian (including human) tissues. Betaine is formed in cells as an oxidation product of choline and can be obtained in the diet from foods such as spinach and beets.
Though data on betaine is limited, and recent, the available literature suggests that this compound may have effects in a few areas. Studies on betaine using servings as little as 1.25g/day and up to 5g/day for up to 14 days have shown promising results. In one study, a 2.5g/day serving was found to enhance endurance and total repetition volume for the squat, bench press, and jump squat in healthy-exercised trained adults. A similar study using the same serving found that betaine use increased peak power and maximum peak power, along with force and the maintenance of both force and power in healthy, exercise-trained subjects.
Perhaps more interesting, however, is a study which examined betaine’s effect on the endocrine system. This study revealed that betaine may exert an effect on several endocrine processes given the proper conditions, causing the authors to hypothesize that long(er) term betaine supplementation may increase the hypertrophic response to resistance training.
N-Acetyl L-Carnitine
L-carnitine is a derivative of the amino acid lysine and, as certain conditions outpace the body’s ability to produce it, l-carnitine is considered a conditionally essential amino acid. While endogenous biosynthesis of l-carnitine from the amino acids lysine and methionine is sufficient for essential processes – along with dietary sources of carnitine from protein-rich red meat, for example – dietary supplementation of carnitine may pose benefits in certain physiological conditions.
Unfortunately, due to excess metabolism of l-carnitine by microorganisms in the small intestine, exogenous supplementation with oral l-carnitine has proved ineffective. ALCAR, an acetylated version of l-carnitine, has considerably higher oral bioavailability, due likely to only partial hydrolytic metabolism. Once in the bloodstream, ALCAR plays a fundamental role in the production of energy, acting as the catalyst for the beta-oxidation of long chain fatty acids by the mitochondria; regulating the CoA to Acyl-CoA ratio (necessary for the production of ATP); and the metabolism of carbohydrates. ALCAR also is an excitatory agent for neurons, increases neuronal transmission, and increases the production of neurotransmitters and neurohormones such as dopamine and serotonin.
Enfinity® Paraxanthine
Caffeine is widely known as being the world’s most popular dietary supplement ingredient. It provides energy and wakefulness through a process of inhibiting phosphodiesterase and adenosine. What most people don’t know however is, is that caffeine is made up of three powerful metabolites one metabolized by the liver, Theobromine, Theophylline, and Paraxanthine. Paraxanthine is the star of the show here as it does a large majority of the heavy lifting for caffeine’s felt effects, such as lipolysis, as well as the actual dopamine upregulation and feel-good effects we experience when taking caffeine. Paraxanthine also has stronger binding potencies for adenosine as well as can increase processing speed, improve response time, and promote more sustained attention during activities.
One of the most attractive qualities about paraxanthine is that it has a substantially shorter half-life than both theophylline and theobromine. At 3.1 hours, it’s less than half of the half-life, which leads to caffeine’s general half-life being around 4.1 hours. The one thing about caffeine in general is that its metabolism varies across genetic and ethnic distributions, so not everybody is going to react the same to the same amount of caffeine at the same time taken. In fact, there are fast, medium, and slow metabolizers of caffeine. From person to person, this can mean that caffeine’s half-life can vary anywhere from 1.5-10.5 hours. However, some research has shown that caffeine clearance can vary as much as 40x between consumers. What anecdotal evidence shows when all these individuals ingest paraxanthine, is that it leads to a much smoother and consistent experience for everyone. The shorter half-life works great for slow metabolizers too, especially without them having to worry about the consequences of theobromine and theophylline lingering around. Although studies on paraxanthine are preliminary, the evidence thus far points to the substance being a reliable replacement to traditional caffeine for those who are widely affected by caffeine’s addictive and often negative side effects.
VasoDrive-AP® (Isoleucyl-prolyl-proline (IPP) and Valyl-prolyl-proline (VPP) (from hydrolyzed milk casein)
VasoDrive-AP® is a proprietary ingredient derived from fermented casein. The fermentation process produces two lactotripeptides, Valyl-Prolyl-Proline (VPP) and Isoleucyl-Prolyl-Proline (IPP). Based on the available clinical evidence, these tri-peptides work together to reduce angiotensin converting enzyme (ACE).
In a recent meta-analysis researchers found 30 studies – all of which were randomized and some of which were double-blind – wherein Valyl-Prolyl-Proline (VPP) and Isoleucyl-Prolyl-Proline (IPP), either alone or in combination, exerted a statistically significant effect on improving endothelial function. In one of the analyzed studies, 25 men were challenged with casein hydrolysate standardized for tri-peptides in a randomized and placebo-controlled design. At the conclusion of the study, forearm blood flow, a key measure of endothelial function, was found to be increased by 33%. The researchers in this and other studies demonstrate that the tri-peptides that constitute VasoDrive-AP® exert an inhibitory effect on ACE. ACE, in turn, is a critical component of the renin-angiotensin (RAS) system, which regulates hemodynamics by controlling plasma fluid volume. While the precise mechanism has not been elucidated, researchers believe that the lactotripeptides in VasoDrive-AP® competitively inhibit ACE, thus decreasing the metabolism of bradykinin and systematically dilating the arteries and veins.
Cognizin® Citicoline
Choline is an essential nutrient involved in numerous metabolic pathways, including DNA regulation and repair, protein function, and metabolism. Perhaps most importantly, the critical neurotransmitter acetylcholine is produced directly from free choline via cholinergic neurons. Acetylcholine is then responsible for several functions itself, most crucially as the compound which induces muscular contraction, and as the neuromodulator partially responsible for modulating risk/reward, arousal, and enhancing memory.
Choline’s essential role as a substrate for acetylcholine, and therefore brain development, is well documented in animal models. These studies demonstrate that levels of free maternal choline have a direct and fundamental impact on prenatal brain development, with the enhancements or deficits lasting into adulthood. Choline’s enhancing effect is particularly prominent in the hippocampus. In humans, the hippocampus is primarily involved in the consolidation of memory (taking short, episodic memory and translating it into long-term memory) and the learning of new information. Acetylcholine is a critical component in these processes, as mentioned above, and choline may therefore play a potential role in these processes as well by providing the substrate for acetylcholine synthesis.
Citicoline (Cytidine 5’-diphosphocoline), also known as CDP-choline, is a potentially superior form of choline due to its ability to cross the blood brain barrier. In fact, most studies with neurological or nootropic effects used this form. In that regard, studies in otherwise healthy, normal adults demonstrated meaningful and statistically significant impacts on working memory, recall, and attention. We have chosen to use the clinically tested, Cognizin®, in this premium formula as our primary choline source. Unlike other synthetic stimulants that can cause a rapid decline in effectiveness after an initial burst of energy, Cognizin® can offer critical nutritional support for the brain that can help support needed focus and attention.
Senactiv® (Panax notoginseng (Root) & Rosa roxburghii (Fruit))
Senactiv®, derived from extracts of Panax notoginseng and Rosa roxburghii, has been shown to act in a few pathways to reenergize and rejuvenate cells within the body that take a beating daily. Ultimately this product falls within a category known as senolytics, or compounds that primarily work to induce cell death. This may not sound ideal as we need our cells to function, but the key is to ensure that these compounds are working on the right kinds of cells. The human body does contain many senescent cells, which are old cells and can deteriorate. How these senolytics work to promote health is by speeding up the breakdown of these older cells as well as encouraging the grow of new, vibrant cells. The other benefit of Senactiv® and its ingredients is its ability to protect against muscle damage. Ginseng has been shown to help reduce the proinflammatory state that is associated with rigorous training. Where this is going to benefit is to reduce inflammation and jumpstart the recovery process of torn down muscle fibers so these cells can begin to scar and grow stronger. The other benefit that ginseng has been able to show is it has been shown to improve VO2 max by about 20% when compared to a control group in studies. This improvement can significantly improve oxygen uptake to increase delivery to muscle cells for the ability to push harder and longer.
On another angle of recovery, Senactiv® has been shown to improve the rate of glycogen storage after exercise. When glycogen can be stored efficiently post exercise it could attenuate the structural damage associated with training by allowing those carbohydrates and sugars to go towards recovery.
Senactiv® is a unique blend of ingredients but with sound research and application behind but with its benefits towards improved cell regeneration, VO2 max, and recovery, it is a great addition to this hydration product.
Huperzia serrata Extract (1% Huperzine A)
Huperzia serrata is a compound found in the plant families of Huperziaceae, Lycopodiaceae, and Selaginella and is endemic to China. The Lycopodium alkaloid Huperizine-A, found in UNMATCHED DISSIDENT, was first isolated from a folk medicinal preparation in 1984.
Due to the potent anticholinesterase activities of Huperzine A, the compound has been evaluated in numerous in vitro, in vivo, and human trials. These data suggest that Huperzine’s Ache activities are most potent in the cortex, hippocampus, and striatum (at least in rats) – key regions in the brain responsible for forming, coordinating, and recalling memory. These effects are assisted by Huperzine A’s high oral bioavailability. Studies using microdialysis technique in rats, for example, showed that the response to Huperzine A was dose-dependent and substantially lowered the level of ACh in cortex.
Huperzine A has also shown promise in humans. Used as a reversible inhibitor of acetylcholinesterase, Huperzine A has shown positive benefits on cognition and as a therapy for individuals suffering from Alzheimer’s Disease. This is partly due to its inhibitory factors on acetylcholine but also its neuroprotective properties. In another study on memory and learning performance, 34 pairs of middle school students complaining of memory inadequacy were given a small dose of Huperzine A. The students were then match paired along several vectors and provided tests on working memory. The results of this study exhibited that HupA markedly improved the memory function of adolescent students.
REFERENCES
Hetzler, R. K., et al. “Effect of Paraxanthine on FFA Mobilization after Intravenous Caffeine Administration in Humans.” Journal of Applied Physiology (Bethesda, Md.: 1985), vol. 68, no. 1, 1 Jan. 1990, pp. 44–47, pubmed.ncbi.nlm.nih.gov/2312486/, doi:10.1152/jappl.1990.68.1.44; https://pubmed.ncbi.nlm.nih.gov/2312486/
Benowitz, Neal L., et al. “Sympathomimetic Effects of Paraxanthine and Caffeine in Humans*.” Clinical Pharmacology & Therapeutics, vol. 58, no. 6, Dec. 1995, pp. 684–691, doi:10.1016/0009-9236(95)90025-x; https://pubmed.ncbi.nlm.nih.gov/8529334/
Bromberg-Martin, Ethan S et al. “Dopamine in motivational control: rewarding, aversive, and alerting.” Neuron vol. 68,5 (2010): 815-34. doi:10.1016/j.neuron.2010.11.022; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3032992/
Wardle, Margaret C et al. “Caffeine increases psychomotor performance on the effort expenditure for rewards task.” Pharmacology, biochemistry, and behavior vol. 102,4 (2012): 526-31. doi:10.1016/j.pbb.2012.06.016; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3578395/
Orrú, Marco, et al. “Psychostimulant Pharmacological Profile of Paraxanthine, the Main Metabolite of Caffeine in Humans.” Neuropharmacology, vol. 67C, 1 Apr. 2013, pp. 476–484, doi:10.1016/j.neuropharm.2012.11.029; https://www.sciencedirect.com/science/article/abs/pii/S002839081200576X
Guerreiro, Serge, et al. “Paraxanthine, the Primary Metabolite of Caffeine, Provides Protection against Dopaminergic Cell Death via Stimulation of Ryanodine Receptor Channels.” Molecular Pharmacology, vol. 74, no. 4, 11 July 2008, pp. 980–989, doi:10.1124/mol.108.048207; https://pubmed.ncbi.nlm.nih.gov/18621927/
Ren, Xiangpeng, and Jiang-Fan Chen. “Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms.” Frontiers in neuroscience vol. 14 602697. 17 Dec. 2020, doi:10.3389/fnins.2020.602697; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7773776/
Orrú, Marco et al. “Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans.” Neuropharmacology vol. 67 (2013): 476-84. doi:10.1016/j.neuropharm.2012.11.029; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3562388/
Nehlig, Astrid. “Is Caffeine a Cognitive Enhancer?” Journal of Alzheimer’s Disease, vol. 20, no. s1, 14 Apr. 2010, pp. S85–S94, pubmed.ncbi.nlm.nih.gov/20182035/, doi:10.3233/jad-2010-091315; https://pubmed.ncbi.nlm.nih.gov/20182035/
Yoo, Choongsung et al. “Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial.” Nutrients vol. 13,11 3980. 9 Nov. 2021, doi:10.3390/nu13113980; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8622427/
Wadhawan, Manav, and Anil C. Anand. “Coffee and Liver Disease.” Journal of Clinical and Experimental Hepatology, vol. 6, no. 1, 1 Mar. 2016, pp. 40–46, doi:10.1016/j.jceh.2016.02.003; https://pubmed.ncbi.nlm.nih.gov/27194895/
Heath, Ryan D, et al. “Coffee: The Magical Bean for Liver Diseases.” World Journal of Hepatology, vol. 9, no. 15, 28 May 2017, pp. 689–696, doi:10.4254/wjh.v9.i15.689; https://pubmed.ncbi.nlm.nih.gov/28596816/
Gressner, Olav A., et al. “Identification of Paraxanthine as the Most Potent Caffeine-Derived Inhibitor of Connective Tissue Growth Factor Expression in Liver Parenchymal Cells.” Liver International, vol. 29, no. 6, July 2009, pp. 886–897, doi:10.1111/j.1478-3231.2009.01987.x; https://pubmed.ncbi.nlm.nih.gov/19291178/
Volkow, N D et al. “Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain.” Translational psychiatry vol. 5,4 e549. 14 Apr. 2015, doi:10.1038/tp.2015.46; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4462609/
Solinas, Marcello, et al. “Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens.” The Journal of Neuroscience, vol. 22, no. 15, 1 Aug. 2002, pp. 6321–6324, doi:10.1523/jneurosci.22-15-06321.2002; https://www.jneurosci.org/content/22/15/6321
dePaula, Juliana, and Adriana Farah. “Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks.” Beverages, vol. 5, no. 2, 1 June 2019, p. 37, doi:10.3390/beverages5020037; https://www.mdpi.com/2306-5710/5/2/37/htm
Guessous, Idris, et al. “Associations of Ambulatory Blood Pressure with Urinary Caffeine and Caffeine Metabolite Excretions.” Hypertension, vol. 65, no. 3, Mar. 2015, pp. 691–696, doi:10.1161/hypertensionaha.114.04512; https://pubmed.ncbi.nlm.nih.gov/25489060/
Stavric, B. “Methylxanthines: Toxicity to Humans. 3. Theobromine, Paraxanthine and the Combined Effects of Methylxanthines.” Food and Chemical Toxicology, vol. 26, no. 8, Jan. 1988, pp. 725–733, doi:10.1016/0278-6915(88)90073-7; https://pubmed.ncbi.nlm.nih.gov/3058562/
Okuro, Masashi et al. “Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice.” Sleep vol. 33,7 (2010): 930-42. doi:10.1093/sleep/33.7.930; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2894435/
Barnes, Peter J. “Theophylline”. American Journal of Respiratory and Critical Care Medicine. Volume 188, Issue 8. 03 May 2013. Doi: 10.1164/rccm.201302-0388PP; https://www.atsjournals.org/doi/full/10.1164/rccm.201302-0388PP
Purpura, Martin, et al; “Paraxanthine-based bioactive composition and method of use thereof”. United States Patent and Trademark Office. Patent US20230072854A1. 9 Mar. 2023; https://patents.google.com/patent/WO2021151094A1/
Wittayalertpanya, Supeecha, et al. “Paraxanthine/Caffeine Ratio: As an Index for CYP1A2 Activity in Polycyclic Aromatic Hydrocarbons Exposed Subjects.” Journal of the Medical Association of Thailand = Chotmaihet Thangphaet, vol. 86 Suppl 2, 1 June 2003, pp. S310-317; https://pubmed.ncbi.nlm.nih.gov/12930004/
Silveri MM et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. 2008; 21(10):1066-75.
McGlade E. et al. Improved Attentional Performance Following Citicoline Administration in Healthy Adult Women. Food and Nutrition Sciences. 2012;3:769-773.
McGlade E, et al. The Effect of Citicoline Supplementation on Motor Speed and Attention in Adolescent Males. Journal of Attention Disorders. 2015; 1557-1246.
Nakazaki E, et al., J Nutr. 2021 Aug 7;151(8):2153-2160.
Fioravanti, M., & Buckley, A. E. (2006). Citicoline (Cognizin) in the treatment of cognitive impairment. Clinical interventions in aging, 1(3), 247–251. https://doi.org/10.2147/ciia.2006.1.3.247Babb SM, et al. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology (Berl). (2002)
Silveri MM, et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. (2008)
PNAS, Sep 20 2005, 102(38):13681-13686. L-citrulline and L-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits.
Br J Clin Pharma, 2008, 65:51-59 Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism.
Urology, Jan 2011, 77(1):119-22. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction
Tangphao O, et al. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. (1999).
Curis E, Crenn P, Cynober L. Citrulline and the gut. Curr Opin Clin Nutr Metab Care. (2007).
Bahri S, et al. Mechanisms and kinetics of citrulline uptake in a model of human intestinal epithelial cells. Clin Nutr. (2008).
Takeda K, et al. Effects of citrulline supplementation on fatigue and exercise performance in mice. J Nutr Sci Vitaminol (Tokyo). (2011)
Drozak J, et al Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1) . J Biol Chem. (2010)
Miyaji T, et al Expression profiles of carnosine synthesis-related genes in mice after ingestion of carnosine or ß-alanine . J Int Soc Sports Nutr. (2012)
Chez MG, et al Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders . J Child Neurol. (2002)
Hipkiss AR On the enigma of carnosine’s anti-ageing actions . Exp Gerontol. (2009)
Boldyrev AA Does carnosine possess direct antioxidant activity . Int J Biochem. (1993)
Hipkiss AR, Michaelis J, Syrris P Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent . FEBS Lett. (1995)
Dutka TL, Lamb GD Effect of carnosine on excitation-contraction coupling in mechanically-skinned rat skeletal muscle . J Muscle Res Cell Motil. (2004)
Bauer K, Schulz M Biosynthesis of carnosine and related peptides by skeletal muscle cells in primary culture . Eur J Biochem. (1994)
Dunnett M, Harris RC Influence of oral beta-alanine and histidine supplementation on the carnosine content of the gluteus medius . Equine Vet J Suppl. (1999)
Kennedy D. O. (2016). B Vitamins and the Brain: Mechanisms, Dose and Efficacy--A Review. Nutrients, 8(2), 68. https://doi.org/10.3390/nu8020068
Tang L. L., Wang R., Tang X. C. (2005). Effects of huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells. Acta Pharmacol. Sin. 26, 673–67810.
Tuszynski M. H., Sang H., Yoshida K., Gage F. H. (1991). Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann. Neurol. 30, 625–63610.
Wang C. Y., Zheng W., Wang T., Xie J. W., Wang S. L., Zhao B. L., et al. (2011). Huperzine A activates Wnt/β-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 36, 1073– 108910.
Wang L. M., Han Y. F., Tang X. C. (2000). Huperzine A improves cognitive deficits caused by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 398, 65–7210. Wang R., Xiao X. Q., Tang X. C. (2001a). Huperzine A attenuates hydrogen peroxideinduced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. Neuroreport 12, 2629–2634.
Wang R., Zhang H. Y., Tang X. C. (2001b). Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by beta- amyloid protein-(1–40) in rat. Eur. J. Pharmacol. 421, 149–15610.1016/S0014-2999(01)01030-5. Wang X. D., Zhang J. M., Yang H. H., Hu G. Y. (1999).
Modulation of NMDA receptor by huperzine A in rat cerebral cortex. Acta Pharmacol. Sin. 20, 31–35. Wang Y. E., Yue D. X., Tang X. C. (1986). Anti-cholinesterase activity of huperzine A. [Article in Chinese]. Zhongguo Yao Li Xue Bao 7, 110–113.
Lever M, Sizeland PC, Bason LM, Hayman CM, Chambers ST. Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta1994;1200:259–64.
Sakura N, Ono H, Nomura S, Ueda H, Fujita N. Betaine dose and treatment intervals in therapy for homocystinuria due to 5, 10-methylenetetrahydrofolate reductase deficiency. J Inherit Metab Dis1998;21:84–5.
Virtanen E, Junnila M, Soivio A. Effects of food containing betaine/amino acid additive on the osmotic adaptation of young Atlantic salmon, Salmo salar L. Aquaculture 1989;83:109–22.
Bloomer RJ, Farney TM, Trepanowski JF, McCarthy CG, Canale RE. Effect of betaine supplementation on plasma nitrate/nitrite in exercise-trained men. J Int Soc Sports Nutr. 2011 Mar 18;8:5.
Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004; 80: 539–549. Hoffman JR, Ratamess NA, Kang J, Rashti SL, Faigenbaum AD. Effect of betaine supplementation on power performance and fatigue. J Int Soc Sports Nutr. 2009 Feb 27;6:7.
Hoffman JR, Ratamess NA, Kang J, Gonzalez AM, Beller NA, Craig SA. Effect of 15 days of betaine ingestion on concentric and eccentric force outputs during isokinetic exercise. J Strength Cond Res. 2011 Aug;25(8):2235-41.
Lee EC, Maresh CM, Kraemer WJ, Yamamoto LM, Hatfield DL, Bailey BL, Armstrong LE, Volek JS, McDermott BP, Craig SA. Ergogenic effects of betaine supplementation on strength and power performance. J Int Soc Sports Nutr. 2010 Jul 19;7:27.
Ortiz-Costa S, Sorenson MM, Sola-Penna M (2002) Counteracting effects of urea and methylamines in function and structure of skeletal muscle myosin. Arch Biochem Biophys 408:272–278
Pryor JL, Craig SA, Swensen T. Effect of betaine supplementation on cycling sprint performance. J Int Soc Sports Nutr. 2012 Apr 3;9(1):12.
Trepanowski JF, Farney TM, McCarthy CG, Schilling BK, Craig SA, Bloomer RJ. The effects of chronic betaine supplementation on exercise performance, skeletal muscle oxygen saturation and associated biochemical parameters in resistance trained men. J Strength Cond Res. 2011 Dec;25(12):3461-71.
Lee, T., Wu, J., Jean, W. H., Condello, G., Alkhatib, A., Hsieh, C. C., Hsieh, Y. W., Huang, C. Y., & Kuo, C. H. (2021). Reduced stem cell aging in exercised human skeletal muscle is enhanced by ginsenoside Rg1. Aging, 13(12), 16567–16576. https://doi.org/10.18632/aging.203176
Pumpa KL, Fallon KE, Bensoussan A, Papalia S. The effects of Panax notoginseng on delayed onset muscle soreness and muscle damage in well-trained males: a double blind randomised controlled trial. Complement Ther Med. 2013 Jun;21(3):131-40.
Martin-Rincon M, Morales-Alamo D, Calbet JAL. Exercise-mediated modulation of autophagy in skeletal muscle. Scand J Med Sci Sports. 2018 Mar;28(3):772-81.
Li QJ, Nan Y, Qin JJ, Yang Y, Hao XJ, Yang XS. [Chemical constituents from medical and edible plants of Rosa roxburghii.] Zhongguo Zhong Ya Za Zhi. 2016 Feb;41(3):451-5.
Liang MTC, Podolka TD, Chuang WJ. Panax notoginseng supplementation enhances physical performance during endurance exercise. J Strength Cond Res. 2005 Feb;19(1):108-14.
Liu MH, Zhang Q, Zhang YH, Lu XY, Fu WM, He JY. Chemical analysis of dietary constituents in Rosa roxburghii and Rosa sterilis fruits. Molecules. 2016 Sep9;21(9):1204.
Kim DH. Chemical diversity of Panax ginseng, Panax quinquefolium, and Panax notoginseng. J Ginseng Res. 2012 Jan;36(1):1-15.
Turpeinen, A.; Kumpu, M.; Rönnback, M.; Seppo, L.; Kautiainen, H.; Jauhiainen, T.; Vapaatalo, H.; Korpela, R. Antihypertensive and cholesterol-lowering effects of a spread containing bioactive peptides IPP and VPP and plant sterols. J. Funct. Food 2009, 1, 260–265.
Turpeinen, A.; Ikonen, M.; Kivimaki, A.S.; Kautiainen, H.; Vapaatalo, H.; Korpela, R. A spread containing bioactive milk peptides Ile-Pro-Pro and Val-Pro-Pro, and plant sterols has antuhypertensive and cholesterol-lowering effects. Food Funct. 2012, 3, 621–627.1
Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Korpela, R. Lactobacillus helveticus fermented milk reduced arterial stiffness in hypertensive subjects. Int. Dairy J. 2007, 17, 1209– 1211.
Usinger, L.; Ibsen, H.; Linneberg, A.; Azizi, M.; Flambard, B.; Jensen, L. Human in vivo study of the renin-angiotensin-aldosterone system and the sympathetic activity after 8 weeks daily intake of fermented milk. Clin. Physiol. Funct. Imaging 2010, 30, 162–168.
Itakura, H.; Ikemoto, S.; Terada, S.; Konodo, K. The effect of sour milk on blood pressure in untreated hypertensive and normotensive subjects. J. Jpn. Soc. Clin. Nutr. 2001, 23, 26–31.
Hirota, T.; Ohki, K.; Kawagishi, R.; Kajimoto, Y.; Mizuno, S.; Nakamura, Y.; Kitakaze, M. Casein hydrolysate containing the antihypertensive tripeptides Val-Pro-Pro and Ile-Pro-Pro improves vascular endothelial function independent of blood pressure-lowering effects: Contribution of the inhibitory action of angiotensin-converting enzyme. Hypertens. Res. 2007, 30, 489–496.
Yasuda, K.; Aihara, K.; Komazaki, K.; Mochii, M.; Nakamura, Y. Effect of large intake of tablets containing “lactotripeptides (VPP, IPP)” on blood pressure, pulse rate and clinical parameters in healthy volunteers. J. Nutr. Food 2001, 4, 63–72.
Ishida, Y.; Sagitani, A.; Kaneko, K.; Nakamura, Y.; Mizutani, J.; Masuda, O.; Watanabe, M.; Sato, S.; Shioya, N. Antihypertensive effects of the tablet containing “lactotripeptide (IPP, VPP)” in subjects with high normal blood pressure or mild hypertension. J. Pharmacol. Ther. 2007, 35, 1249–1260























